On October 1, 2025, the McIntyre Medical Building hosted an engaging Mini-Symposium & Discussion Day in Rooms 210/11 at 3655 Promenade Sir William Osler.
The event followed the McGill–Kyoto–ASHBi Symposium on Advances in Human Biology and Their Significance for Genomic Medicine*, continuing the momentum of cross-institutional scientific exchange on the next day October 2nd 2025
Throughout the day, participants enjoyed a series of short presentations delivered by joint McGill–Kyoto PhD students, highlighting the strong collaborative research efforts emerging from the partnership between the two universities. Their talks showcased innovative projects at the intersection of human biology and genomic medicine.
Dr. Satoshi Yoshiji CERC PI, Dr. Sirui Zhou CERC PI, Ms. Mayumi Yoshida CERC Project administrator, and Dr. Vincent Mooser, CERC Chair, were also in attendance, providing support to CERC and contributing to the vibrant atmosphere of interdisciplinary discussion and knowledge sharing.
Highlights of the Day
The sessions covered a wide range of topics, including:
Single-cell and spatial genomics
Epigenetics and CRISPR technologies
Long-read sequencing and RNA innovations
Distinguished Speakers
The symposium featured renowned experts from McGill University and Kyoto University, including:
Mitinori Saitou (ASHBi, Kyoto University)
Mark Lathrop (McGill University)
Yasuaki Hiraoka (ASHBi, Kyoto University)
Claudia Kleinman (McGill University)
Yue Li (McGill University)
Raquel Cuella Martin (McGill University)
Knut Woltjen (Kyoto University)
Taka Inoue (ASHBi, Kyoto University)
Yasuhiro Murakawa (ASHBi, Kyoto University)
Ioannis Ragoussis (McGill University)
Thomas Duchaine (McGill University)
CERC related Presentations
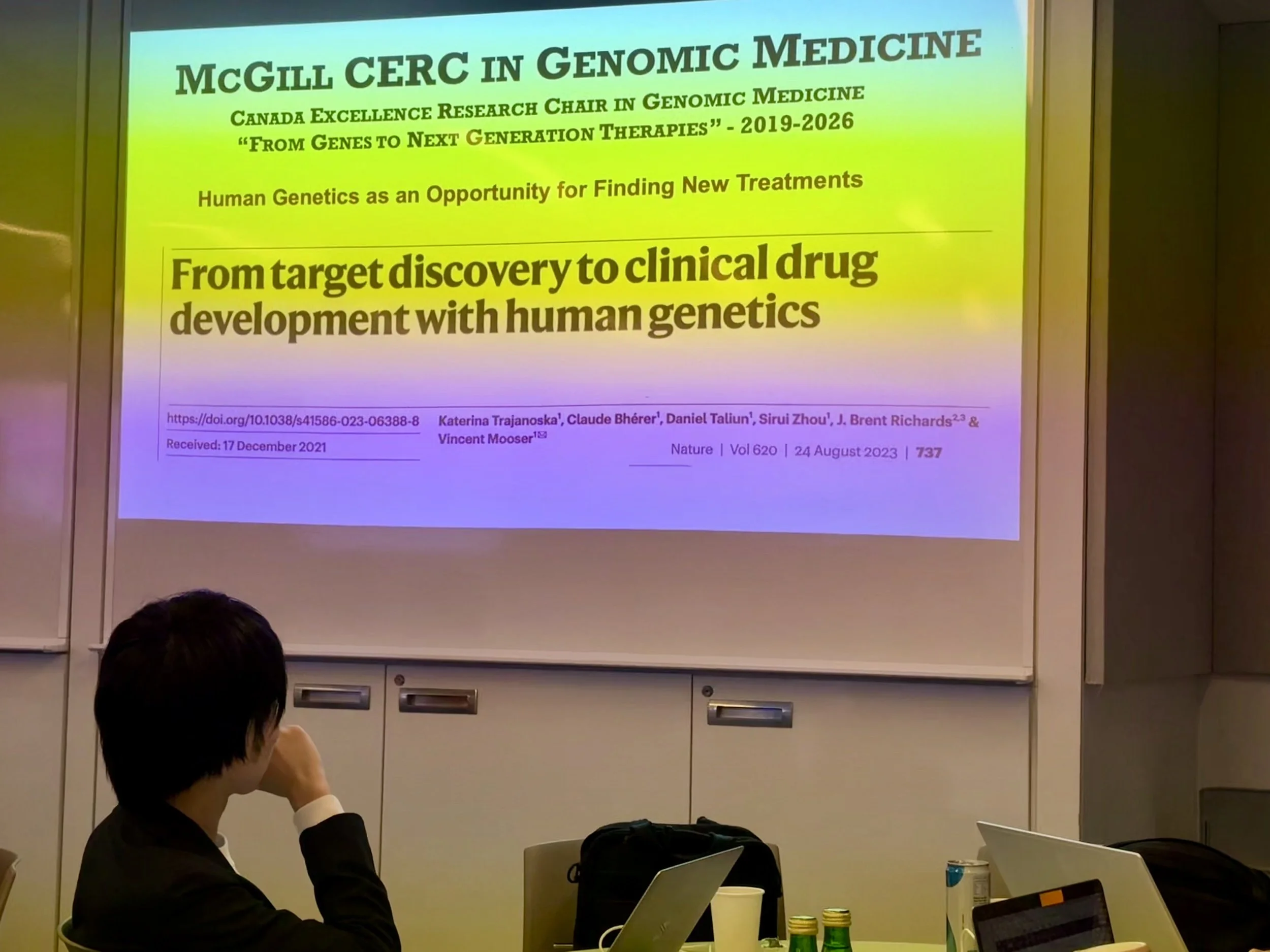
Dr. Mark Lathrop, Scientific Director, Victor Phillip Dahdaleh Institute of Genomic Medicine, shared insights into CERC activities, highlighting strategic initiatives and collaborative opportunities.
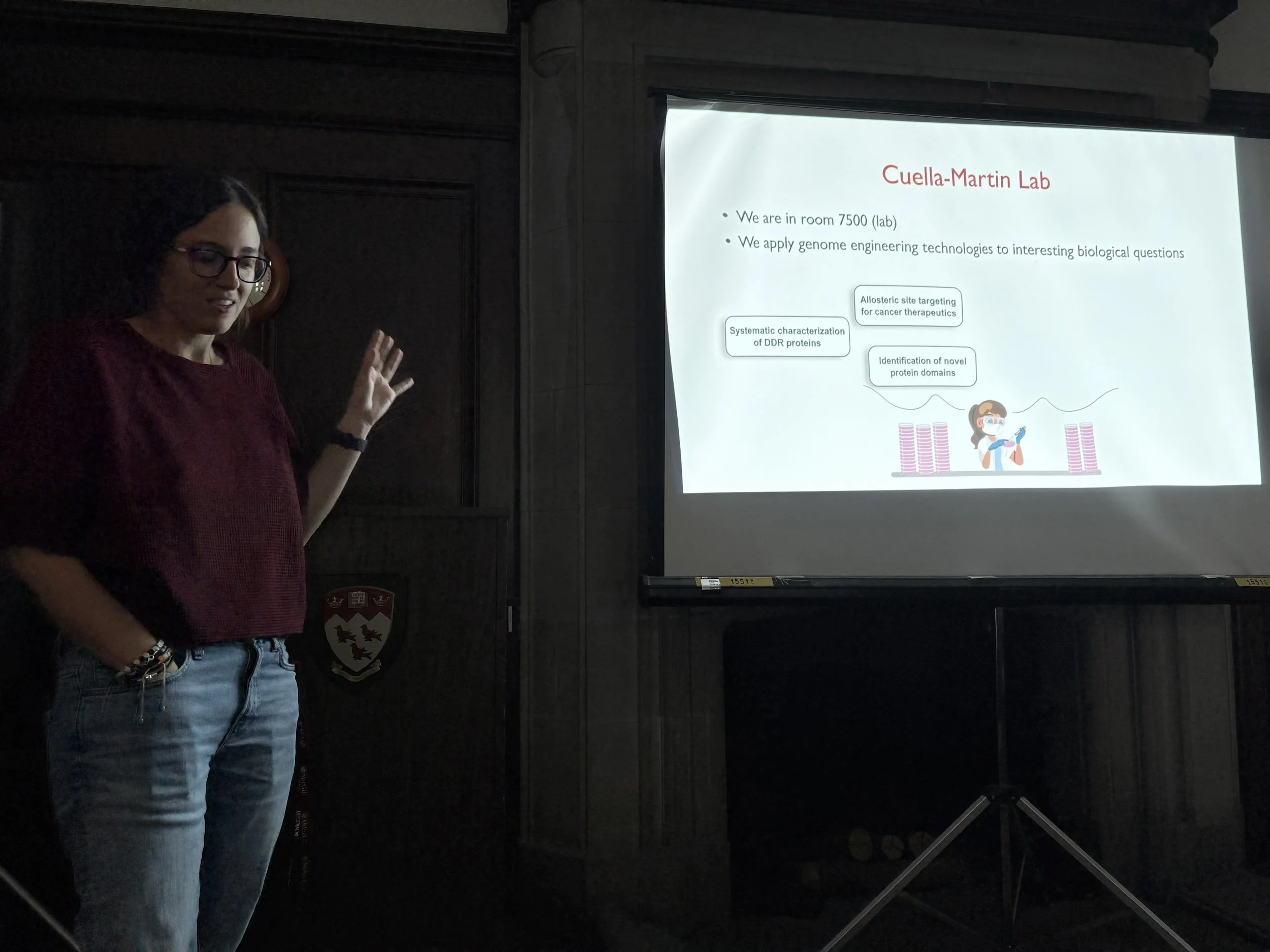
Dr. Raquel Cuella Martin, CERC PI, presented her research alongside her team, offering an in-depth look at their latest findings.
Two joint McGill–Kyoto PhD students, Susannah Selber- Hnaiw from Dr. Sirui Zhou lab and Masashi Hasebe from Dr. Saotshi Yoshiji Lab, delivered compelling presentations on their projects.
Networking Reception
The day concluded with a cocktail reception at the Redpath Museum (859 Sherbrooke Street West, Montreal), offering attendees an informal setting to connect with speakers and collaborators.