Vincent Mooser MD, Canada Excellence Research Chair (CERC) in Genomic Medicine, McGill, Montreal Brent Richards MD PhD, PI of BIO-PORTAL, Lady Davis Institute, Jewish General Hospital, Montreal David Morrison, BIO-PORTAL Manager, Lady Davis Institute, Jewish General Hospital, Montreal
Premise
The demand for clinical data paired with genomics is at an all-time high. Drug discovery programs supported by human genetics are 2-4-fold more likely to reach the market. Similarly, precision, i.e. molecularly- (including genetically)-enriched, drug development programs open up an unprecedented opportunity to accelerate and inform critical proof-of-concept (PoC) studies and prioritize early development programs for a number of assets which are coming up to humans.
BIO-PORTAL Vision, Mission and Strategy
BIO-PORTAL is a unique research platform at the Jewish General Hospital (JGH)/Lady Davis Institute in Montreal built in partnership with the CERC Chair in Genomic Medicine at McGill. BIO-PORTAL is fully aligned with the JGH strategy in digital health and is designed to address industry needs in large quantity of real-world, high-quality clinical data and to access specific sub-populations for their clinical trials.
Overall, BIO-PORTAL vision is to transform clinical care by applying the latest knowledge and technologies in genomics and IT to benefit all patients admitted and who are provided care at the JGH and, ultimately, to the entire administrative population the JGH belongs to (called CIUSSS Centre-Ouest).
BIO-PORTAL mission is to leverage a bank of biomedical information from thousands of patients to incorporate precision medicine and genomics into clinical decision-making and deliver more effective, precise care to patients.
From a strategic perspective, BIO-PORTAL will build on the experience gained in designing, building and exploiting the largest single-site COVID-19 biobank in the country, within the framework of the Québec COVID-19 Biobank (BQC19, https://en.quebeccovidbiobank.ca). BIO-PORTAL will now focus on improving care for diabetes and will then rapidly expand to other common and rare diseases. Our teams will initially collect the health information in electronic medical records and biological samples from 2,500 diabetic patients per year. With artificial intelligence, our researchers will analyze this information to better understand the individual’s genetic risk for disease and its complications, clarify their diagnoses and find better medicines for their clinical conditions. Later, these individuals will be re-contacted for clinical trials that will test the application of this research to patient care and have them benefit from the latest therapeutics. Using an open-science approach, qualified researchers and industry investigators will be able to access our data to help guide our teams to the best possible clinical options. This BIO-PORTAL resource will also provide a large pool of patients to draw from for investigator-led, industry-sponsored trials.
Distinct Population, Unrivaled Clinical Data, Open Data, Unique Consent
What differentiates BIO-PORTAL from other biobanks? Firstly, the deep clinical datasets we have been able to produce in collaboration with Digital Health. Second, the distinct and diverse populations that the JGH serves. Third, open access to data. Fourth, REB-approved consent which enables recall-by-genotype studies.
Deep Phenotypic Data
Over the past two years, the BIO-PORTAL team in collaboration with Digital Health, has built one of the richest hospital-based datasets for COVID19. Types of data include ER visits, admission, ICU admission, vitals on arrival, administered medications in hospital, pharmacy reconciliation, previous medical history by ICD10 code, consult history, procedures performed, all lab values, microbiology, CT/XR/MRI imaging, and blood type. For the diabetes project, phenotypes will include, on top of those listed above, retinal images using AI-assisted technologies, FibroScan to document liver damage and, under consideration, brain imaging to document small vessel diseases.
Founder/Diverse Populations
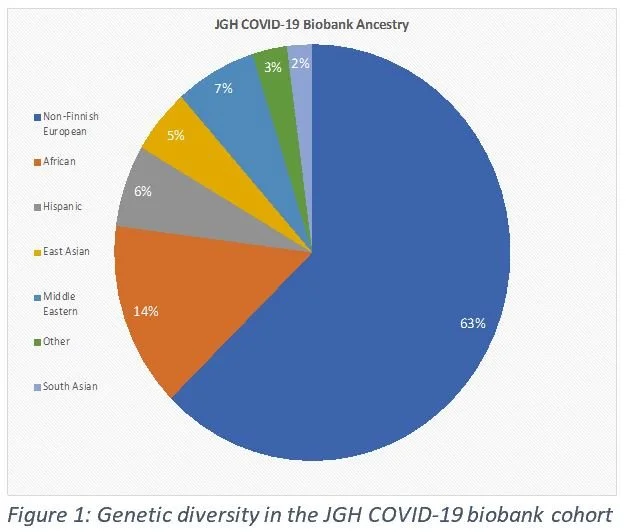
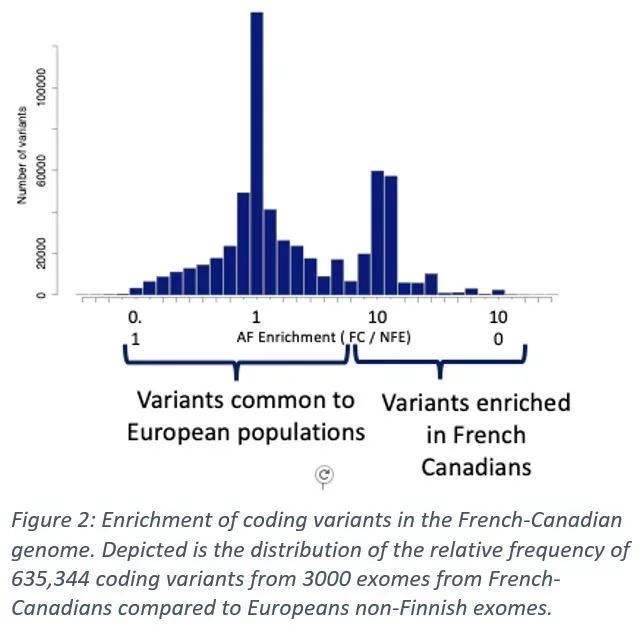
In Quebec, we are fortunate to have founder populations (Charlevoix/St-Jean) and other closed or somewhat closed communities (Hasidic, Italian, Greek, etc.). The area of Cote-Des-Neiges has one of the highest percentages of non-European ancestry individuals on the Montreal island (Figure 1). As a comparison, the UK Biobank is a 94% European ancestry. Founder populations coupled with diverse ethnic population provides users with unique variants that appear with a much higher frequency here than elsewhere in the world (Figure 2).
Open Access
Unlike most other biobanks (BioVU, FinnGen, Geisinger), BIO-PORTAL will be open-access2, meaning any group who has REB approval and Data Access Committee endorsement will be given access to the data. This is good for science and good for us as it increases our user base, our scientific productivity and national and international visibility.
Consent allowing recall-by-genotype studies
Unlike most other biobanks, BIO-PORTAL has already designed and implemented a REB-approved consent allowing recall-by-genotype (or other molecular biomarkers), allowing partnerships with industry to accelerate their early- and late-stage development programs.
Addressing the needs of researchers
Access to large quantity of real-world high-quality data from patients diagnosed with diabetes and related metabolic disorders
Access to such patients for selected, molecularly enriched early development clinical trials
Access to biological material from such patients
Access to data, samples and patients diagnosed with specific rare diseases.